Stabilizing Protein Drugs with a New Reversible “Mixing-type” Material

Researchers develop a novel “supermolecular” material that improves the stability and efficacy of protein drugs
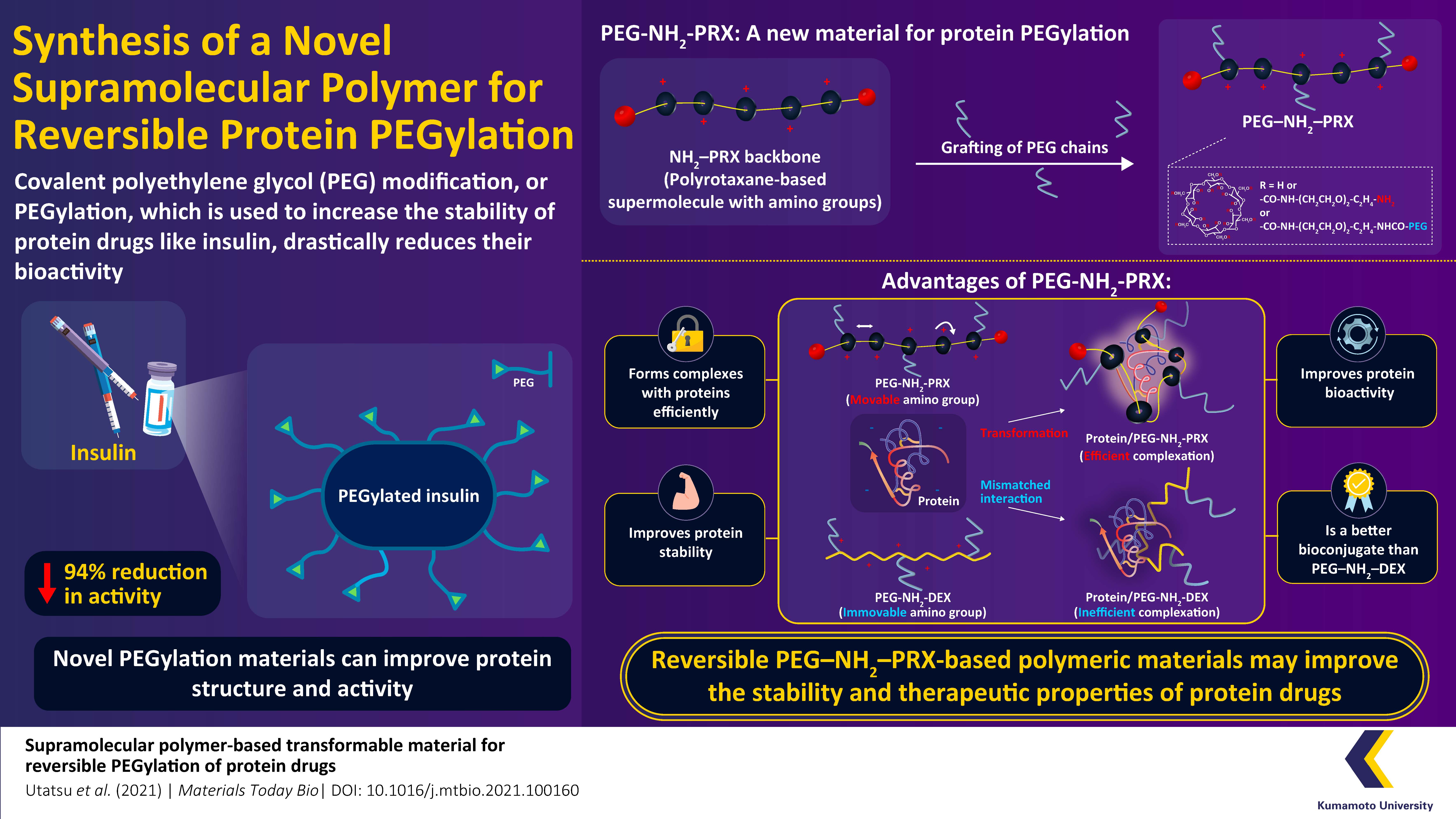
Protein drugs are widely used as pharmaceutical agents to treat a range of disorders. However, most of these drugs are not very stable and are only retained in the blood circulation for a limited period, which minimizes their efficacy. To resolve these concerns, researchers at Japan's Kumamoto University have developed a new material that binds successfully to protein drugs and prolongs the duration of their effect without impairing their activity, thereby improving overall drug performance.Recombinant protein drugs have recently gained popularity in the treatment of several clinical conditions, including cancer, infections, and genetic abnormalities. Under physical stress, these drugs often have limited stability, which can impair their biological action, or “bioactivity.” Another issue emerges when these drugs are not retained in the bloodstream and are quickly eliminated from circulation. Following significant research, scientists devised a method of adding polyethylene glycol (PEG), a polymer produced from petroleum, to proteins, in an effort to extend a protein drug's sustainability in blood and also prevent clumping. Unfortunately, tight binding of PEG chains interferes with the proteins, resulting in reduced or complete loss of drug activity. For a long time, this drug chemistry baffled scientists worldwide, fostering research on the development of removable and reversible PEGylation materials that can be added and removed as and when required.
Carrying on this trend, in their new study made available online on 16 November 2021 and published in Volume 12 of Materials Today Bio, Associate Professor Taishi Higashi and his team of researchers at Kumamoto University used the compound “polyrotaxane” (PRX) to create a new “supramolecular” material that could reversibly add PEG chains to proteins without impairing their activity. First, they created a reversible agent called “PEG-PRX” by mixing PEG with PRX, and then coupled it with insulin to form the “PEG-PRX-Insulin” complex. When administered to diabetic rats, the complex maintained the effect of insulin without compromising its activity.
The group had previously reported another PEGylation material created using cyclodextrin. However, the steric hindrance caused by the PEG chains in this earlier study allowed the protein to be easily removed causing low drug retention. “We assumed that the weak interaction was due to the mismatch in shapes and charge distributions. Therefore, this time we decided to develop a reversible PEG agent that deforms according to the shape and charge of the protein and interacts strongly with it,” explains Dr. Higashi. “When the PRX molecule with a “positive charge” is mixed with a protein with a “negative charge” such as insulin, the complex becomes both flexible and efficient. This flexibility appears to be critical for the efficient assembly of complexes," he adds.
To ascertain the material’s flexibility, the authors compared their PRX derived experimental outcomes to those of a less flexible agent, dextran (DEX). In the PRX versus DEX comparisons, it was fairly clear that the PRX interactions were much stronger and kept the insulin more tightly bound in the complex. This increased both the stability and availability of the drug. When asked if these comparative differences could be due to the varying chemical compositions of both materials, Dr. Higashi clarifies, “Importantly, the molecular weights of PRX and DEX complexes in our experiments were almost the same, suggesting their performance was not influenced by the difference in their chemical characteristics.”
Motivated by their initial findings, the researchers turned their attention to a different class of protein drugs called “antibody drugs,” which have recently gained popularity as first-line treatment modalities for different cancer types but are impacted by aggregation during transportation and storage. Aggregation leads to the loss of antibody activity resulting in serious side effects. By conducting shaking stress experiments, the authors confirmed that adding the novel PEG-PRX agent to antibodies resulted in no aggregate formation after 7 days, whereas the control group aggregated 90% of the antibodies! “We obtained excellent results not just with the standard but also with a commercial antibody drug, suggesting that our material has great potential to be a stabilizing agent for these medicinal agents,” exclaims Dr. Higashi.
Given the complexity of therapeutic proteins and the wide range of biological materials used in their production, it is extremely important to improve their functional characteristics while retaining safety and durability. The team not only investigated the efficacy of their newly produced material, but also confirmed its safety profile in healthy rats. Dr. Higashi and his colleagues are confident about the diverse therapeutic prospects of their findings. "Because this supramolecular material can ameliorate the flaws of diverse protein drugs simply by mixing them together, we expect it will be used as a pharmaceutical material with both efficacy and flexibility," Dr. Higashi concludes.
[Funding]
- The Naito Foundation
- Cooperative Research Project of the Research Center for Biomedical Engineering
- Leading Initiative for Excellent Young Researchers
[Usage Restrictions]
The authors request that a reference to this release be included when using the media contained within this release elsewhere.