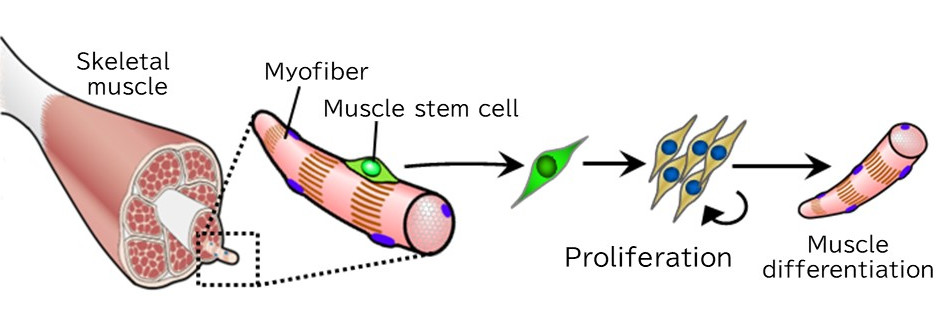
Muscles retain positional memory from fetal life

New perspectives on the pathological mechanisms of muscle diseases and regenerative medicine development.
A research collaboration based in Kumamoto University, Japan has discovered that muscles and the resident stem cells (satellite cells) responsible for muscle regeneration retain memory of their location in the body. This positional memory was found to be based on the expression pattern of the homeobox (Hox) gene cluster, which is responsible for shaping the body during fetal life. These findings are expected to provide clues to elucidate the pathogenesis of muscle diseases such as muscular dystrophy, in which the position of muscle vulnerability varies depending on the type of muscle, and to help develop regenerative medicine based on positional memory.There are various types of the intractable muscle disease muscular dystrophy and each type has a different symptom location. Similarly, age-related muscle fragility (sarcopenia) does not occur evenly throughout the body. The physical location of the symptoms of these diseases cannot be explained by differences in muscle fiber types or physical activity patterns alone, and requires a new perspective to elucidate their respective pathogeneses.
The developmental origin of cells that form muscles differ in the fetal stage. For example, most of the craniofacial muscles originate from the cranial mesoderm, while the limb muscle
Using skeletal muscle and associated muscle stem cells isolated from the heads and hind limbs of adult mice, researchers investigated positional specificity at the epigenomic level using DNA methylome analysis. They found characteristic differences in the DNA methylation status at the homeobox (Hox) loci. Among four regions, A to D, the Hox-A locus in particular had an overall DNA hypermethylation state in hindlimb skeletal muscle and muscle stem cells compared to the head. Additionally, both skeletal muscle and muscle stem cells in the hind limbs showed high expression of the Hox-A gene. Many of these Hox-A genes reflected expression patterns in the fetal period. These findings suggest that skeletal muscle and muscle stem cells remember positional information during fetal life, and that epigenomic regulation by DNA methylation may be involved in positional memory.
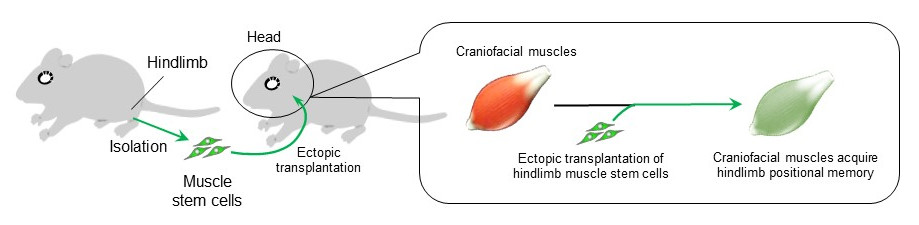
The researchers then focused on the Hoxa10 gene, which was highly expressed only in the limb muscles. When hindlimb-derived muscle stem cells expressing Hoxa10 were isolated and transplanted into craniofacial muscles that do not express Hoxa10, Hoxa10 gene expression became detectable in the craniofacial muscles. In other words, hindlimb-derived muscle stem cells were able to innervate the craniofacial muscle with strong retention of positional memory even after ectopic transplantation.
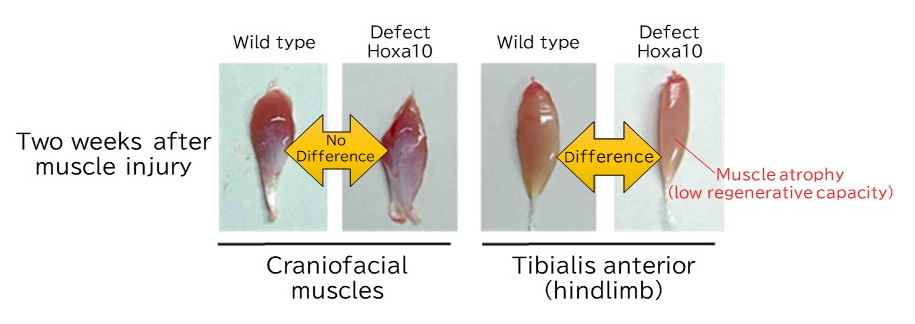
They then created mice lacking the Hoxa10 gene in muscle stem cells to analyze its function. A Hoxa10 deficiency severely impaired the regeneration of hindlimb muscles but had no effect on craniofacial muscle regeneration. A detailed investigation of the mechanism behind the hindlimb muscle regeneration disorder revealed that it is caused by genomic instability due to abnormal chromosome distribution during muscle stem cell division. Furthermore, analysis of human head and leg muscle stem cells also showed that only leg muscle cells expressed the HOX-A gene and that its inhibition resulted in abnormal cell division, confirming that muscle cell positional memory is retained in humans and mice.
This research suggest that the positional memory of muscle stem cells based on the position-specific distribution of Hox gene expression may determine the position-specific properties of skeletal muscle, rather than merely persisting from fetal life.
"In the future, we expect that the functional aspects of muscle stem cell positional memory will lead to the clarification of the mechanisms that lead to location-specificity of symptoms that are observed in various muscle diseases like muscular dystrophy," said Associate Professor Yusuke Ono, who led the study. "In addition, ectopic transplantation experiments, in which muscle stem cells are transplanted to a location different from where they were harvested, have shown that they maintain positional memory and regenerate. From a different perspective, skeletal muscles regenerated from xenotransplantation may not possess their original positional information which may impair their normal function. There has been rapid progress recently in the differentiation of iPS cells into various progenitor cells and the development of mass culture techniques, but the location of induced progenitor cells has not been considered. In the future, our group will attempt to develop regenerative therapy applications for muscle diseases by artificially controlling the positional memory of cells and by utilizing the properties of cells with positional memory in the right places."
This research was posted online in Science Advances on 9 June 2021.
[Source]
Yoshioka, K., Nagahisa, H., Miura, F., Araki, H., Kamei, Y., Kitajima, Y., ... Ono, Y. (2021). Hoxa10 mediates positional memory to govern stem cell function in adult skeletal muscle. Science Advances, 7(24), eabd7924. doi:10.1126/sciadv.abd7924
[Funding]
- Agency for Medical Research and Development (AMED) Japan (16bm0704010, 19bm0704036, 18ek0109383),
- The Grants-in-Aid for Scientific Research KAKENHI from MEXT Japan (16J09948, 17H03608, 18H03193, 18K19749, 20J01490, 20K19537, 20H00456),
- The Takeda Science Foundation,
- Japanese Physical Therapy Association,
- The Platform Project for Supporting Drug Discovery and Life Science Research [Basis for Supporting Innovative Drug Discovery and Life Science Research (BINDS)] from AMED Japan (JP20am0101103)
Image credits belong to Associate Professor Yusuke Ono unless otherwise indicated in the figure caption. To use any of the media contained within this release elsewhere, a reference to the original work (this release) should be included and other restrictions must be followed as indicated.