Unveiling the Role of FOXO1 in Vascular Development and Transcriptional Dynamics in Endothelial Cells

With extended vascular endothelial growth factor (VEGF) exposure, FOXO1 translocates to the nucleus in Tip cells, guiding vascular branching
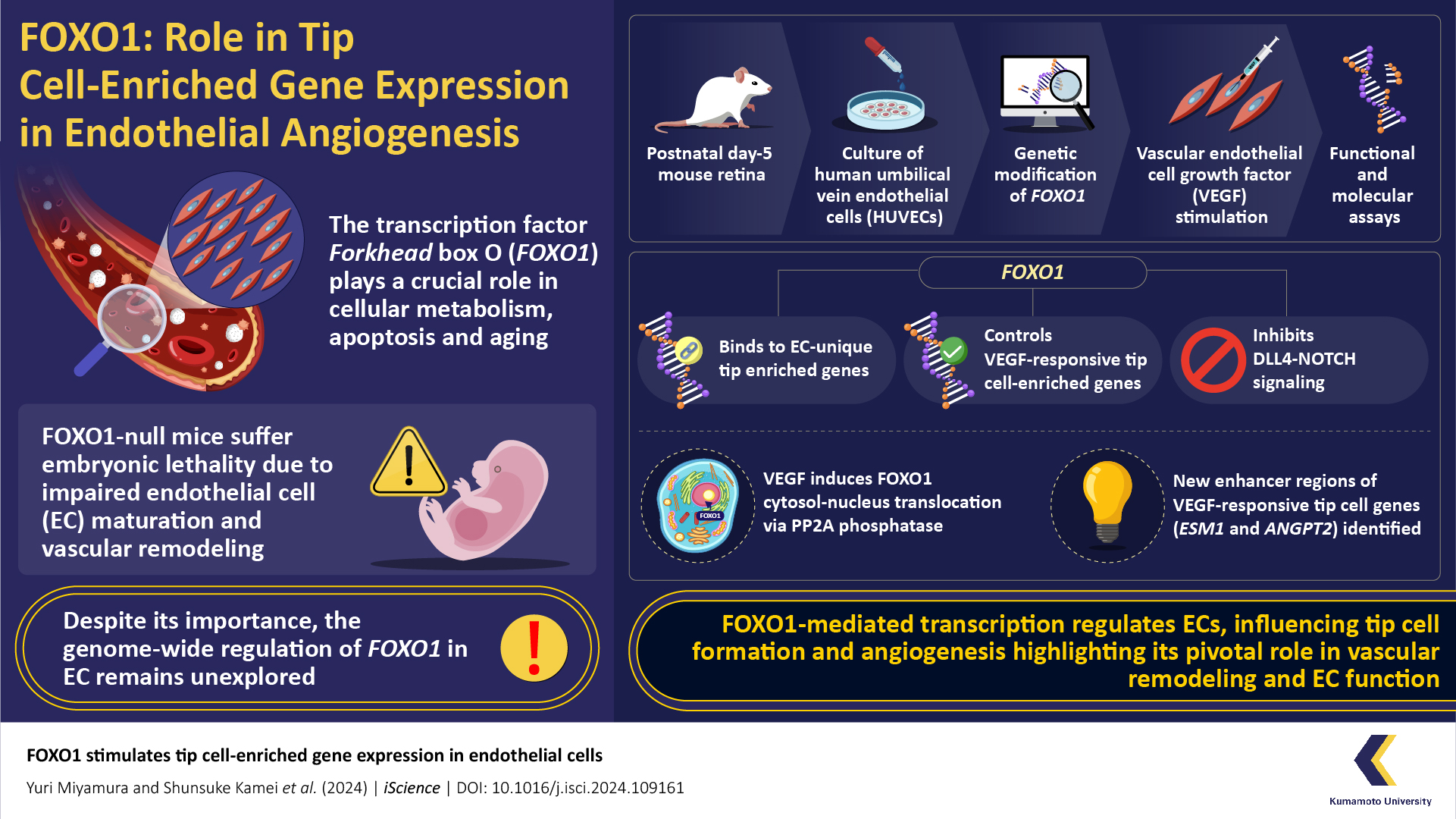
FOXO1, a key transcription factor in cellular processes, governs metabolism, apoptosis, and aging. Despite its significance, the comprehensive regulation of FOXO1 in endothelial cells (EC) remains unexplored. A recent study under the leadership of Professor Takashi Minami from Kumamoto University identified crucial factors involved in the branching of blood vessels and revealed the transcriptional system of Tip-Stalk dynamics in ECs during vascular endothelial growth factor (VEGF) stimulation.
The human vascular system, a complex network of blood vessels, plays an essential role in maintaining health. Understanding the molecular mechanisms underlying vascular development is important for tackling age-related disorders. The Forkhead box O (FOXO1) transcription factor is crucial in processes related to aging, cellular metabolism, and apoptosis. Despite its significance, the comprehensive regulation of FOXO1 across the genome in endothelial cells (EC) has not been investigated.
In a new paper published in iScience in February 2024, a research team from Kumamoto University employed advanced techniques, including next-generation sequencing to comprehensively analyze vascular ECs stimulated by the potent vascular endothelial growth factor (VEGF) and were subjected to a comprehensive analysis. This allowed the team to observe sequential changes in the cellular localization of FOXO1, providing unique insights into its dynamic role during prolonged VEGF stimulation. The corresponding author of the paper, Professor Takashi Minami explains, "We uncovered cell type-specific functions of FOXO1, shedding light on its role in VEGF-mediated Tip cell definition in primary cultured endothelial cells. Our study explores novel pathways for understanding FOXO1's functions in cellular processes, offering insights into vascular biology."
The researchers focused on blood vessel development by studying the role of FOXO1 in ECs, examining how FOXO1 influences gene activity during VEGF-induced vessel remodeling. To understand this, the research team used techniques of immunostaining on mouse retina tissues and spheroid assays with human ECs. They observed FOXO1 localization in Tip cells, crucial for blood vessel sprouting. The study also investigated FOXO1 dynamics upon VEGF treatment and chemical inhibitors to understand the involvement of various signaling pathways in this process.
Moreover, using advanced genomic analyses, researchers identified FOXO1-regulated genes involved in vessel maturation. The study's unique approach included ChIP-seq and RNA-seq analyses, providing insights into FOXO1's direct binding to specific gene regions. Professor Minami says, "Recognizing the critical need to understand the mechanism governing the definition of Tip versus stalk cells during sprout maturation and the role of FOXO1 activity in VEGF-mediated vessel remodeling, we conducted a study that assessed FOXO1-regulated genes through single-cell RNA-seq and Chip-seq analyses in VEGF-treated ECs."
The researchers focused on the transcriptional system governing Tip-Stalk determination. Prolonged VEGF stimulation, FOXO1 undergoes a remarkable translocation to the nucleus specifically in Tip cells, a critical phase in vascular branching. This activated state triggers the expression of genes that determine the fate of Tip cells. Simultaneously, FOXO1 inhibits NOTCH signaling in stalk cells, responsible for cell proliferation. Importantly, NOTCH signaling reciprocally hinders FOXO signaling, preventing stalk cells from adopting Tip cell characteristics. FOXO1 shows a distinctive binding to endothelial cells-unique Tip cell-enriched genes. The study also identified new enhancer regions for VEGF-responsive Tip cell genes (ESM1 and ANGPT2).
In conclusion, these findings shed light on FOXO1's crucial role in ECs during blood vessel formation, particularly in the context of VEGF-induced angiogenesis. While Tip cells guide the direction of vascular branching, stalk cells support the growth of the emerging vessels. This finding not only deepens our understanding of vascular remodeling but also emphasizes the cell-specific functions of FOXO1.
Moreover, they provide a comprehensive understanding of the aging-associated decline in vascular health and pave the way for exploring novel mechanisms to counteract vascular diseases such as metastatic cancer and recurrent cardiovascular diseases. Offering the potential to enhance overall health in an aging population and ways to manage diseases in the future, this study proves to be a significant achievement in the field of vascular biology.
About Professor Takashi Minami
Professor Takashi Minami is the head of the Molecular Vascular Control Field. Prof. Minami, formerly a postdoctoral researcher and instructor at MIT and Harvard Medical School's Beth Israel Deaconess Medical Center, brings over 13 years of experience as an associate professor and professor at the University of Tokyo's Center for Advanced Science and Technology, where he led the 'Vascular Biology Laboratory.' He has contributed to 32 research papers and 17 research projects. Prof. Minami specializes in research on the growth and regeneration of blood vessels, as well as studies to elucidate the mechanism of diseases related to blood vessels.