Effect of an Autism-Associated Mutation on Protein Movements

A series of experiments reveal that a germline mutation of the enzyme topoisomerase II B affects the movement of proteins in the nuclei of cells with this mutation
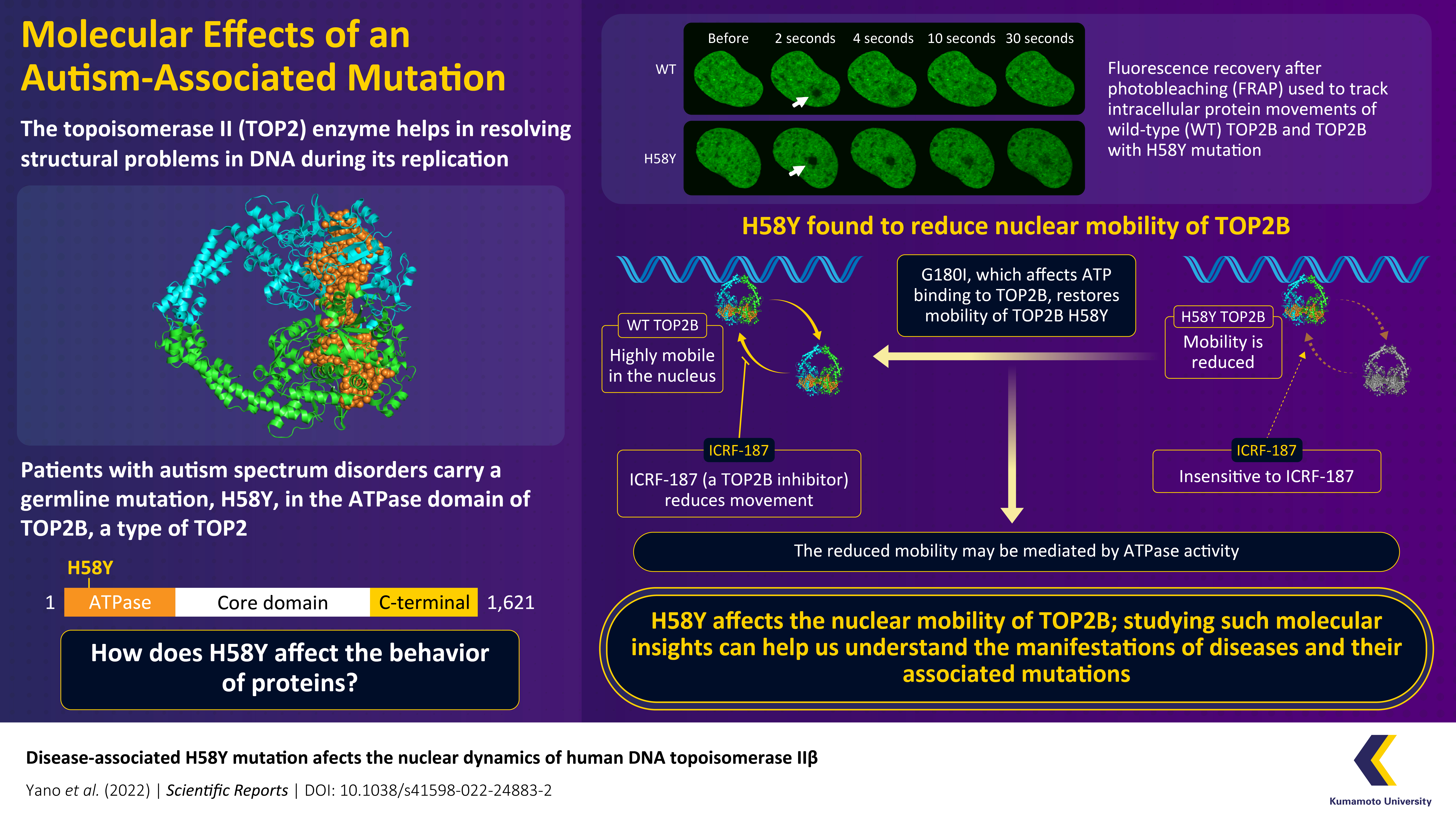
The enzyme topoisomerase II (TOP2) resolves structural issues that arise during DNA replication and transcription, and mutations in this protein manifest as serious diseases. Although there has been significant progress in the discovery of such disease-associated mutations, their functional significance remains unknown. Now, researchers from Japan find that the presence of an H58Y mutation, that is associated with autism, severely impacts the nuclear mobility TOP2B, a subtype of TOP2.
The processes of DNA replication and transcription may introduce torsional (or twisting) strains in the DNA structure. These topological problems are relieved by an enzyme called topoisomerase II (TOP2) via a series of reactions. An important enzyme in this regard, TOP2 has an evolutionarily conserved structure comprising an N-terminal ATPase domain, catalytic core, and a C-terminal domain. Notably, humans have two kinds of TOP2–TOP2A, essential for cell division processes, and TOP2B, an important player in the proliferation and gene expression of neurons.
A series of previous studies revealed that missense mutations in the catalytic domain of TOP2B can lead to deleterious consequences. Recently, in patients with autism spectrum disorder and global developmental delay, a heterozygous germline mutation (i.e., a genetic mutation in a reproductive cell that is later reflected in every cell of the offspring) was discovered in the ATPase domain of TOP2B. In this mutation, histidine is substituted by tyrosine in the 58th position, and therefore, it is called H58Y (henceforth, referred to as TOP2B H58Y) substitution. Interestingly, this mutation does not cause extensive structural changes in TOP2B. So, researchers surmised that it causes a functional change unrelated to TOP2B’s catalytic activity.
In order to understand the biological significance of the H58Y mutation better, a group of researchers led by Prof. Ken-ichi Yano from Kumamoto University, Japan, carried out a comprehensive set of experiments. Their results are published in Volume 12 of Scientific Reports on 30 November 2022. Explaining the motivation behind the study, the Prof. Yano says, “Recent advances in genome analysis have revealed a number of gene mutations associated with diseases. Despite these advancements, however, the effect of these mutations on proteins remains unknown. This study provides an example of how mutations affect protein behavior in cells.”
Fluorescence recovery after photobleaching (FRAP) is a method of monitoring intracellular molecular movements. Using FRAP analysis, the team compared the nuclear dynamics of wild-type TOP2B (TOP2B WT) with TOP2B H58Y. Mobility is an important feature of nuclear proteins, and it is crucial for executing various biological functions. Interestingly, TOP2B H58Y was significantly less mobile in comparison to TOP2B WT.
The reduced mobility of TOP2B H58Y was further confirmed by its insensitivity to an inhibitor of TOP2, ICRF-187, which reduces TOP2 mobility by clamping it to DNA. Although TOP2B H58Y did not react to ICRF-187, its response to a TOP2 poison, etopside, which inhibits proliferation, was similar to TOP2B. This result suggested that the mutant enzyme retained some of its catalytic potential.
The team’s experiments also revealed the mechanism underlying the low protein mobility. They found that G180I, which affects the binding of ATP to TOP2B, could restore the mobility of TOP2B H58Y. These results, therefore, showed that TOP2B H58Y’s restrained movements were mediated by ATPase activity.
These experiments have shed light on a rare example of how a disease-associated mutation could altered nuclear dynamics, thus providing a platform to understand the biological relevance of such mutations. Further experiments are necessary for providing insights into the functional impact of the mutated protein’s reduced mobility, and the molecular processes underlying autism. Optimistic about the significance of their results, Prof. Yano concludes that these experiments “can be an important clue for understanding the cause of autism.”
About Professor Ken-ichi YanoKen-ichi Yano is a Professor at the Institute of Industrial Nanomaterials and the Faculty of Advanced Science and Technology, Kumamoto University, Kumamoto, Japan. He received his Ph.D. from the Tokyo Metropolitan University. Primarily, he works in the fields of functional biochemistry and molecular biology. He has published more than 10 important papers in reputed journals, and several book chapters. He holds memberships in the Institute of Electrical Engineers of Japan, the Molecular Society of Japan, and the Japan Epigenetics Research Group.
[Funding]
- JSPS KAKENHI (19H04271)