Highly Concentrated Silicate Nanosheets

The multifunctional material could be used in catalysts, adsorbents, or even in hydrogen fuel cells.
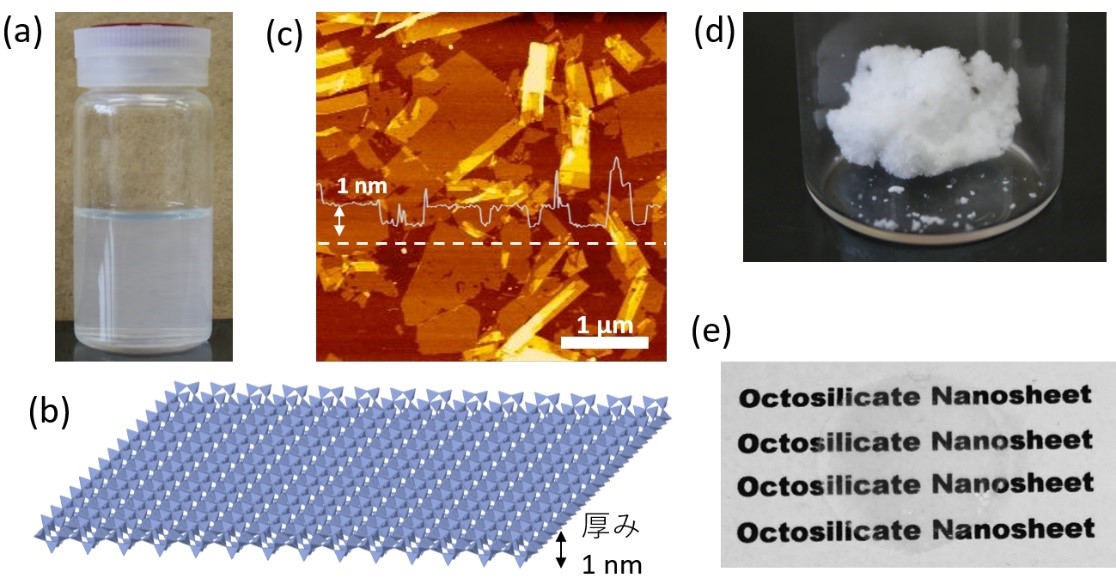
A research collaboration based in Kumamoto University (Japan) has developed a solution of highly concentrated, monolayer dispersion of 1-nanometer-thick silicate (oxygen and silicon) nanosheets. By coating and drying the solution, researchers created transparent silicate nanofilms and freestanding films that are able to function as proton-conducting films for hydrogen fuel cells. Furthermore, they may be considered a sustainable material since they use abundantly available elements.Layered polysilicate compounds have a crystal structure in which the constituent atoms are chained together laterally to form a plate-like layer. This plate-like layer acts as the unit structure and tens to thousands of layers are stacked in the remaining direction. These layered polysilicate compounds have a property called intercalation, which is the reversible incorporation of various ions and molecules between the layers. The distance of interlayer changes is related to the size of the ions and molecules incorporated into the interlayer. Furthermore, when certain substances are used, the distance between the layers expands until the bonds formed between the layers are broken, and each layer (silicate in this case) that formed a layered structure becomes dispersed in the solution.
Silicate materials formed by the exfoliation of such layered structures are also called silicate nanosheets, and methods to produce a dispersed solution of nanosheets have been developed. However, to obtain highly concentrated silicate nanosheets, it is necessary to disperse the layers polysilicate compounds evenly without damaging them which is very difficult. If the dispersion is stable, no precipitates will be deposited in the dispersion solution but if the dispersion is poor, precipitates will be deposited.
In this study, researchers developed a method to effectively exfoliate a layered polysilicate called RUB18, and obtained a highly concentrated dispersed solution of silicate nanosheets. Analysis of the size and thickness of the nanosheets in the solution by atomic force microscopy revealed that the nanosheets were one nanometer thick. When the polysilicate was separated from the dispersion solution, a cottony powder consisting of silicate nanosheets was obtained. Furthermore, researchers found that a transparent silicate nanosheet self-supporting film—a film that maintains its shape without the support of a substrate—could be created if the nanosheets were precisely stacked.
The nanosheets are composed of oxygen and silicon, the first (46.6%) and second (27.7%) most abundant elements in the Earth's crust, making them a promising nanomaterial for the development of alternative materials. They are also chemically stable and have a large surface area which makes them good choices for porous materials and catalysts. The researchers expect that they can also be used in materials that need film-forming, transparent, elastic/flexible, or barrier-forming properties, such as inks, paints, or coating agents.
"We also found that our silicate nanosheets can function as a proton (hydrogen ion) conducting films for hydrogen fuel cells," said Professor Shintaro Ida, who led the study. "Although the power output is low, about 10 microwatts per square centimeter, we expect this can be improved in the near future. It is exciting to think that this material might contribute to a more sustainable society."
This research was published in Chemical Communications on 2 June 2021.
Source:
Awaya, K., Sekiguchi, K., Kitagawa, H., Yamada, S., & Ida, S. (2021). Preparation of silicate nanosheets by delaminating RUB-18 for transparent, proton conducting membranes. Chemical Communications, 57(51), 6304–6307. doi:10.1039/d1cc02110a
[Usage Restrictions]
Image credits belong to Dr. Shintaro Ida unless otherwise indicated in the figure caption. To use any of the media contained within this release elsewhere, a reference to the original work (this release) should be included and other restrictions must be followed as indicated.