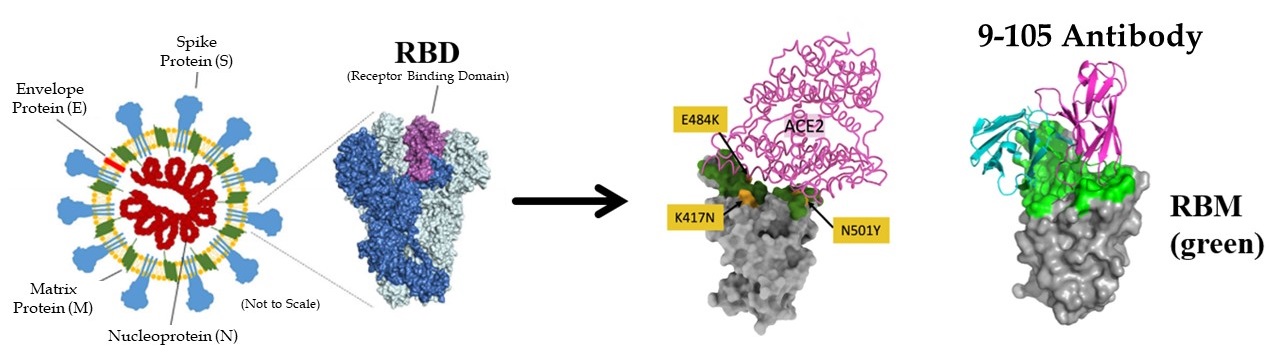
Newly generated monoclonal antibodies effective against SARS-CoV-2 and variants

Japanese researchers have successfully produced potent neutralizing monoclonal antibodies from a patient who recovered rapidly from a severe case of COVID-19. In addition to appearing to have the world's strongest neutralizing activity, the newly developed antibodies have extraordinary binding activity against viral spike proteins. By blocking these bindings, it can prevent infection and reduce the severity of the disease. The antibody was also found to have a neutralizing activity against many variant strains.Among patients with COVID-19 infections, 20% become severely ill and 5% become critically ill, but there is still no effective treatment. Although current vaccines have been effective against the virus so far, variants, like the beta and delta strains, have been reported to be less effective. Furthermore, the pandemic is expected to continue for some time since vaccine effectiveness varies between individuals and some people remain unvaccinated.
In response, a research collaboration based in Kumamoto University, Japan have been working to develop a treatment using neutralizing antibodies to prevent infection and reduce the severity of the disease.
Isolate and identify neutralizing monoclonal antibodies
Two COVID-19 cases with strong neutralizing activity in their serum were selected from patients who recovered after having severe illness. Antibody copies (clones) were made from their peripheral blood B cells, and 88 of the 1,102 clones showed binding activity to the SARS-CoV-2 viral spike protein. Neutralizing activity among these 88 clones was examined using a pseudovirus that expressed the same spike protein as SARS-CoV-2, and five clones showing significant activity were selected.
Evaluation of isolated clone neutralizing activity
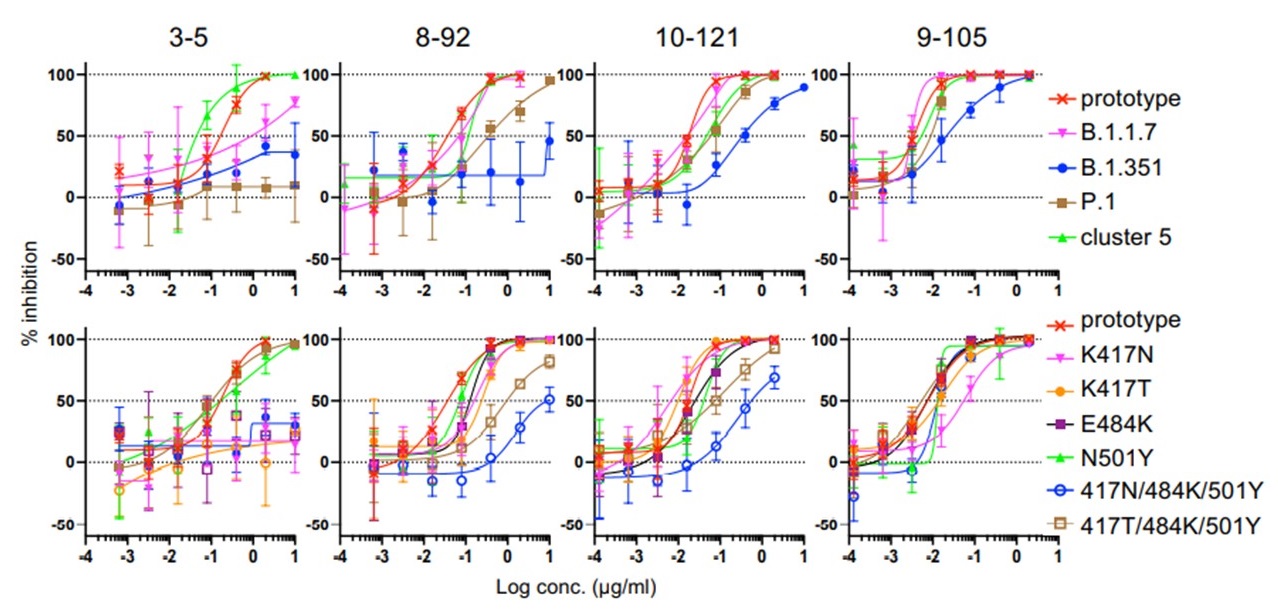
Researchers then examined the neutralizing activity of these five clones (6-74, 3-5, 8-92, 10-121, and 9-105) and found that the 9-105 antibody showed strong neutralizing activity in all tests. The 8-92 and 10-121 antibodies also showed activity comparable to the potent neutralizing antibodies reported previously. Analysis of the binding activity to the receptor binding site (RBD) of the spike protein, the most important site for infection, revealed that the 9-105 antibody had an extraordinarily strong binding activity. In a plaque inhibition study using SARS-CoV-2, the 9-105 antibody also showed strong neutralizing ability.
Evaluation of neutralizing activity against SARS-CoV-2 variant strains
Four important SARS-CoV02 variants are the alpha (B.1.1.7), beta (B.1.351), gamma (P.1), and mink cluster 5 strains. These strains have been reported to be resistant to the antibodies induced by current vaccines and the mink cluster 5 strain also has important implications for the evolution of the virus in animals, which could be the source of the next pandemic strain. The research group created pseudoviruses with the spike protein mutation of each variant and measured antibody cross-neutralizing activity. Strains B.1.1.7 and mink cluster 5 were neutralized by the 9-105, 10-121 and 8-92 antibodies at naturally occurring concentrations. In contrast, variants B1.351 and P.1 were neutralized only by antibodies 9-105 and 10-121, and 9-105 neutralized even the most neutralization-resistant strain, B.1.351, at low concentrations.
Importantly, researchers also found that the 9-105 antibody maintains sufficient neutralizing activity against the delta strain (B.1.617.2), which has become a worldwide problem.
"The COVID-19 pandemic has entered a new phase with the spread of variant strains that are resistant to existing vaccines," said Professor Shuzo Matsushita, the Kumamoto University researcher who led the study. "We have isolated four human monoclonal antibodies with potent neutralizing activities even against variants. Among them, the 9-105 antibody binds strongly to the receptor-binding site of SARS-CoV-2, and is thought to block viral replication even at low concentrations. The neutralizing antibodies approved for emergency use in the U.S. are administered at 70-100 mg/kg, which makes cost an issue. Our work has shown that this potent antibody has a neutralizing activity that is expected to be effective at about 1/100th of that dosage. We believe this will dramatically lower COVID-19 treatment costs around the world."
This research was published in "Cell Reports" on 13 July 2021.
Source:
Kaku, Y., Kuwata, T., Zahid, H. M., Hashiguchi, T., Noda, T., Kuramoto, N., … Matsushita, S. (2021). Resistance of SARS-CoV-2 variants to neutralization by antibodies induced in convalescent patients with COVID-19. Cell Reports, 36(2), 109385. doi:10.1016/j.celrep.2021.109385
[Funders]
- Japan Agency for Medical Research and Development (JP20fk0108271, JP19fk0108111, JP20fk0108270),
- Senshin Medical Research Foundation,
- Ministry of Education, Culture, Sports, Science and Technology Japan (20H05773, 20K20596),
- JST Core Research for Evolutional Science and Technology (20356730),
- JSPS Core-to-Core Program A, the Advanced Research Networks,
- Kumamoto University COVID-19 Research Projects (AMABIE),
- Kumamoto University International Collaborative Research Grants,
- Joint Usage/Research Center program of the Institute for Frontier Life and Medical Sciences Kyoto University
[Usage Restrictions]
Image credits belong to Dr. Shuzo Matsushita unless otherwise indicated in the figure caption. To use any of the media contained within this release elsewhere, a reference to the original work (this release) should be included and other restrictions must be followed as indicated.