New Mechanism Reveals How HTLV-1 Triggers Aggressive Leukemia

Discovery identifies a potential new therapeutic target for adult T-cell leukemiaResearchers at Kumamoto University have uncovered a previously unknown molecular mechanism by which human T-cell leukemia virus type I (HTLV-1) drives the development of adult T-cell leukemia-lymphoma (ATL), one of the most aggressive and difficult-to-treat blood cancers. The findings provide critical insight into why only a small fraction of HTLV-1 carriers develop leukemia—and point toward new strategies for targeted therapy.
HTLV-1 infects an estimated 10 million people worldwide, including approximately 800,000 in Japan. While most carriers remain asymptomatic, about 5% will eventually develop ATL, a disease with limited treatment options and poor prognosis. Understanding the molecular switch that turns viral infection into full-blown cancer has been a long-standing challenge.
The research team, led by Professor Jun-ichirou Yasunaga from Faculty of Life Sciences, Kumamoto University, focused on a viral protein called HTLV-1 bZIP factor (HBZ), which is continuously produced in both HTLV-1–infected cells and ATL cancer cells. Using patient samples and advanced molecular analyses, the researchers discovered a crucial difference: in ATL cells, HBZ accumulates in the cell nucleus, whereas in non-cancerous infected cells it remains largely in the cytoplasm.
The study revealed that this nuclear relocation of HBZ is triggered by activation of the TGF-β/Smad signaling pathway—a pathway known to play complex roles in cancer. The team further identified JunB, a host transcription factor belonging to the AP-1 family, as the key mediator that escorts HBZ into the nucleus. Once there, HBZ and JunB work together to activate genes that promote cancer cell survival and tumor growth.
Importantly, experiments in mouse models showed that suppressing JunB expression significantly reduced tumor formation and growth, directly demonstrating its essential role in ATL progression.
“This study clarifies a critical step in HTLV-1–induced leukemogenesis,” said Professor Yasunaga. “By identifying the TGF-β–HBZ–JunB axis as a driver of ATL, we have revealed a promising new therapeutic target for a disease that urgently needs better treatment options.”
The findings not only deepen scientific understanding of virus-induced cancer but also open new avenues for developing treatments that disrupt this newly identified pathway.
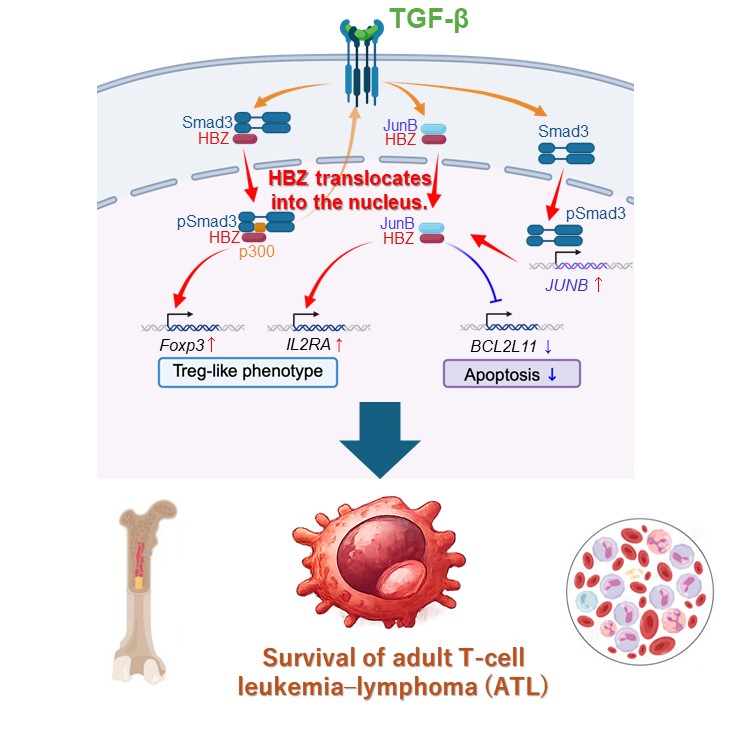
Image Title: TGF-β-Induced HBZ Nuclear Translocation in ATL Cells
Image Caption: This study shows that TGF-β triggers the nuclear translocation of HBZ specifically in ATL cells from patients with ATL, not in HTLV-1–infected cells from asymptomatic carriers. JunB interacts with HBZ, mediates its translocation, and is crucial for ATL cell survival. The HBZ-JunB complex formation in the nucleus is associated with ATL oncogenesis.
Image Caption: This study shows that TGF-β triggers the nuclear translocation of HBZ specifically in ATL cells from patients with ATL, not in HTLV-1–infected cells from asymptomatic carriers. JunB interacts with HBZ, mediates its translocation, and is crucial for ATL cell survival. The HBZ-JunB complex formation in the nucleus is associated with ATL oncogenesis.
Reference
| Authors |
Wenyi Zhang, Takafumi Shichijo, Xueda Chen, Miho Watanabe, Kisato Nosaka, Masao Matsuoka, Jun-ichirou Yasunaga |
| Title of original paper |
JunB-HBZ nuclear translocation by TGF-β is a key driver in HTLV-1-mediated leukemogenesis |
| Journal | Proc Natl Acad Sci USA |
| DOI | 10.1073/pnas.2420756122 |

