Synchronizing Ovulation with Mating Improves Fertilization in “Ultrasuperovulated” Mice

Mating “ultrasuperovulated” mice that produce a high number of oocytes with their breeding pair at the exact time of ovulation or later improves the fertilization rate
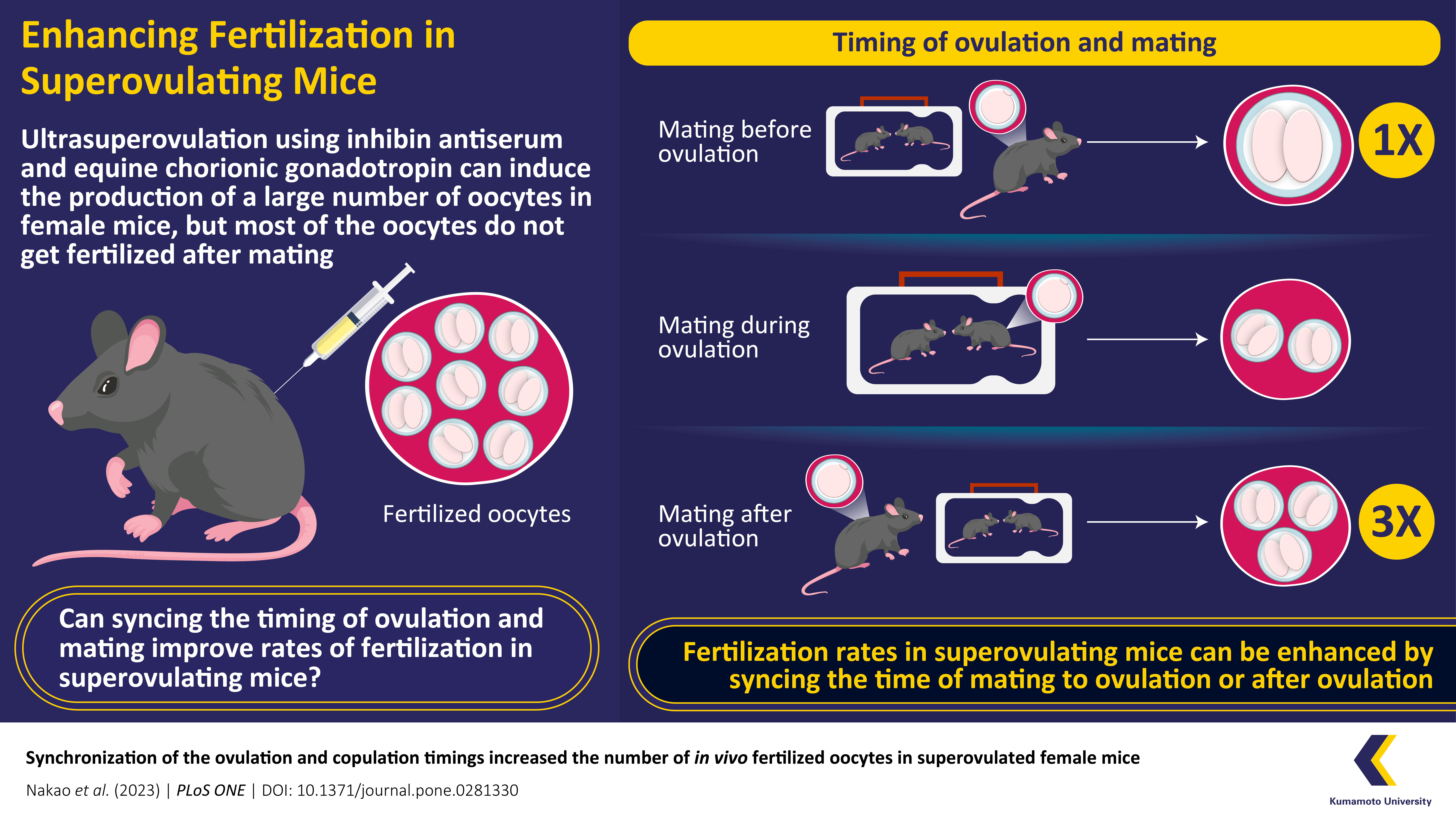
Ultrasuperovulation is an advanced technique that significantly increases the number of oocytes, produced by female mice. However, existing ultrasuperovulation techniques have a low fertilization rate, probably caused by the lack of sperms reaching the female’s oocytes in vivo. Now, a group of researchers from Japan found that mating ultrasuperovulated mice with their breeding partner during or after ovulation can overcome this limitation and improve fertilization rates.
Mice are an indispensable part of experiments in several research fields, including biotechnology and medical science. Since maintaining a breeding strain of mice is essential in most cases, increasing the litter size of these animals is an eagerly pursued goal. Female mice produce about eight to ten oocytes during ovulation. Usually, all the oocytes that are ovulated from the ovaries get fertilized during mating, resulting in a similar number of pups. Now, while scientists have invented techniques to vastly increase the number of ovulated oocytes, these “ultrasuperovulation” techniques fail to produce a similarly large number of fertilized oocytes.
In a study recently published in Volume 18, Issue 2 of PLoS ONE on 6 February 2023, researchers from Japan have demonstrated that synchronizing ovulation and mating can increase the fertilization rate in ultrasuperovulated mice. In the study led by Satohiro Nakao and Kotono Ito of Kumamoto University, researchers divided mice into three groups based on the timing of their ovulation—pre-ovulation, during ovulation, and post-ovulation. Mating female mice with their breeding pair during or after ovulation resulted in a significantly greater number of fertilized oocytes than when they were housed together before ovulation. Professor Toru Takeo, the corresponding author of the paper, who is also affiliated to Kumamoto university, noted that “the number of embryos obtained post-ovulation was three times higher than that obtained pre-ovulation.”
Superovulation is induced in female mice by injecting equine chorionic gonadotropin and human chorionic gonadotropin (hCG). It results in the production of about 20 oocytes and a similar number of fertilized oocytes. Researchers involved in this study had previously discovered an improved technique for superovulation in mice that uses inhibin antiserum and equine chorionic gonadotropin. While this technique, dubbed “ultrasuperovulation”, resulted in over 100 oocytes being released by ovulating mice in each cycle, the number of fertilized oocytes remained around 20. The team hypothesized that the low fertilization rate was caused by a low number of sperms reaching the oocyte in the ampulla, the region of the fallopian tube where fertilization typically occurs. Although millions of sperm reach the uterus during mating, only a tiny proportion swim far enough to reach the ampulla. This can be remedied by timing the mating of mice. According to Prof. Takeo, “The results of this study suggests that a sufficient number of sperm reaches the ampulla to fertilize the oocyte when ovulation and mating are synchronized.”
The time of ovulation induced by superovulation techniques has been accurately established. In the study, the mice in the ‘pre-ovulation’ group were mated 0 to 10 hours, the ‘during ovulation’ group mated 10 to 15 hours, and the ‘post-ovulation’ group mated 15 to 19 hours after receiving an hCG injection.
The underlying physiological mechanism that is responsible for the three-fold increase in fertilization rate demonstrated in the study is not clear. However, Professor Takeo notes, “Synchronizing the ovulation and copulation timings may support the sperm to enter the oviduct and achieve fertilization by riding the oviductal fluid flow; although further studies are required to confirm this phenomenon”.
The findings of the study have several potential practical implications. As mentioned before, a method to increase the reproductive capacity of genetically altered mice that are widely used in scientific research can be highly beneficial. Moreover, the results may help in improving infertility treatments in humans.
About Professor Toru Takeo
Dr. Toru Takeo is currently a Professor at Center for Animal Resources and Development (CARD), Kumamoto University. Professor Takeo has over 21 years of research experience and has published over 81 scientific articles. His academic interests include, among other subjects, Reproductive Technology, Cryobiology and Pharmaceutical Science. Professor Takeo received his PhD from Graduate School of Pharmaceutical Sciences at Kumamoto University in 2008.
[Funding]
- Japan Agency for Medical Research and Development (AMED) Research on Development of New Drugs [Project ID: 16769865]
- Japan Society for the Promotion of Science (JSPS) Grant-in-Aid for Scientific Research (KAKENHI) (C) [Project ID: 20K07015]
- The Cooperative Research Programme from Kyoto University’s Institute for Frontier Life and Medical Sciences