SARS-CoV-2 spike mutation L452R evades human immune response and enhances infectivity

An international team of researchers led by Kumamoto and Tokyo Universities (Japan) have shown that the L452R mutation of the SARS-CoV-2 spike protein, which is common to two mutant strains (Epsilon and Delta), is involved in cellular immunity evasion via the human leukocyte antigen (HLA) A24, and enhances viral infectivity. HLA-A24 is one of the most prominent HLA-class I alleles, especially in East/Southeast Asian populations, which might make them particularly vulnerable to coronavirus variants with this mutation.The ongoing novel coronavirus (SARS-CoV-2 or COVID-19) pandemic has, as of June 2021, infected over 150 million and killed over 3.5 million people worldwide. Vaccination drives around the world are currently underway, but there are still many unknowns, including the principles of infection pathogenesis, the principles viral replication, and the relationship between immune evasion and epidemic dynamics.
Acquired immunity can be broadly classified into humoral immunity mediated by neutralizing antibodies and cellular immunity mediated by helper and killer T cells. SARS-CoV-2 "variants of concern", such as the Alpha and Beta variants, have been studied worldwide for the possibility of humoral immunity evasion. However, cellular immunity evasion has not been reported.
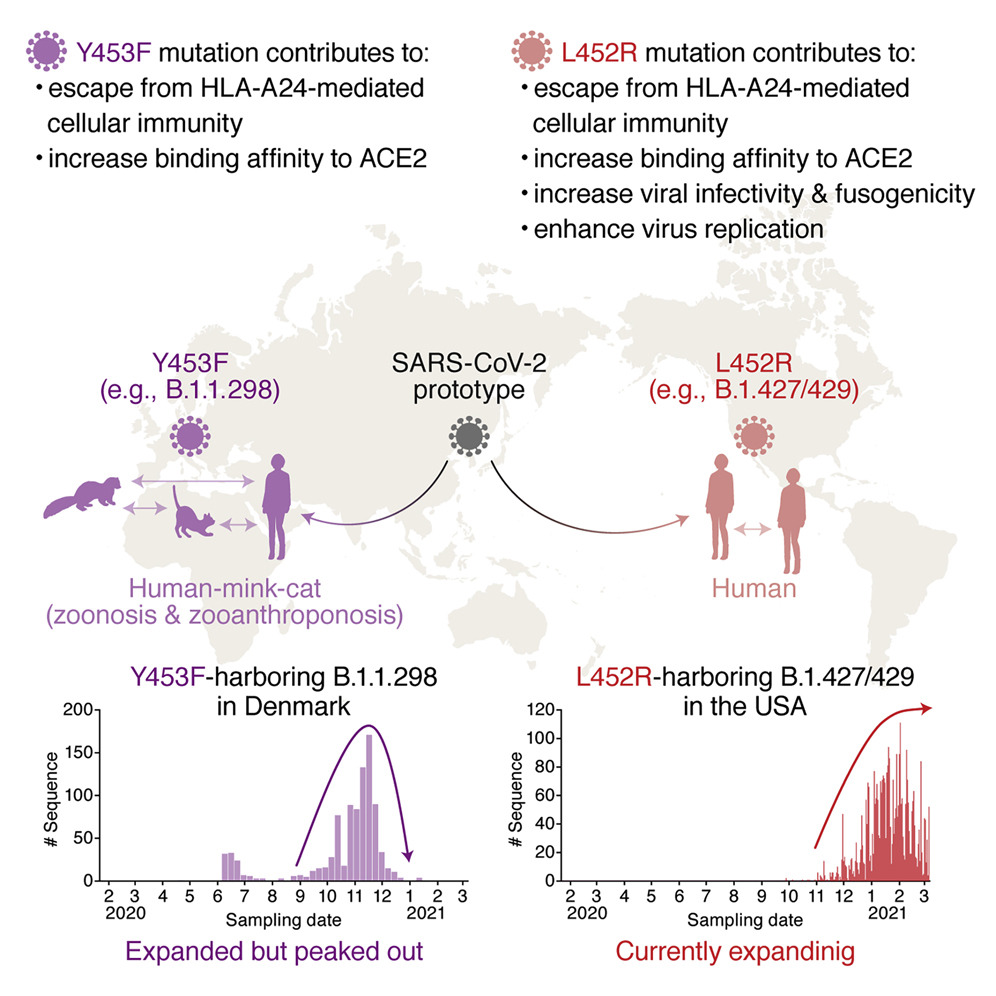
In this study, the research group first used immunological experiments to demonstrate that an antigen derived from the SARS-CoV-2 spike protein is strongly recognized by HLA-A24-restricted cellular immunity, which is often found in Japanese people. They then performed a large-scale (>750,000) sequence analysis of SARS-CoV-2 strains and found several important mutations in the spike protein region typically recognized by HLA-A24. These are the Y453F spike mutations found in strain B.1.1.298, which was prevalent in Denmark in 2020, and the L452R mutation in B.1.427/429 and B.1.617 (commonly known as the Epsilon and Delta variants respectively) that are currently spreading around the world. Further immunological experiments demonstrated that these mutations escape HLA-A24 cellular immunity. The researchers believe that this is the first time a “variant of concern" has been demonstrated to evade cellular immunity.
The Y453F and L452R mutations were located in the receptor binding domain of the SARS-CoV-2 spike protein, which are crucial for gaining entry into host cells. Researchers thus examined the effects of these mutations on the infection and replication efficiency of the virus. They found that the L452R mutation enhances its membrane fusion activity, infectivity, and viral replication.
"The L452R mutation is a hallmark of the Delta variant that is currently spreading worldwide, and in Japan, about 60% of the population have HLA-A24, which is responsible for cellular immunity. The L452R mutation not only evades the HLA-A24 cellular immunity but can also enhance the infectivity of the virus," said the leader of immunology in the study, Dr. Chihiro Motozono." We have been carefully investigating the immune response against emerging SARS-CoV-2 variants in real time to monitor how the mutations affect human immunity and viral infectivity."
This research was posted in Cell Host & Microbe on 14 June 2021.
[Source]
Motozono, C., Toyoda, M., Zahradnik, J., Saito, A., Nasser, H., Tan, T. S., ... Sato, K. (2021). SARS-CoV-2 spike L452R variant evades cellular immunity and increases infectivity. Cell Host & Microbe. doi:10.1016/j.chom.2021.06.006
[Funding]
- AMED Research Program on Emerging and Re-emerging Infectious Diseases (20fk0108163; 20fk0108539; 20fk0108146; 19fk0108171; 20fk0108270; 20fk0108413),
- AMED Research Program on HIV/AIDS (21fk0410046; 20fk0410019; 20fk0410014; 21fk0410039),
- AMED Japan Program for Infectious Diseases Research and Infrastructure (20wm0325009),
- JST A-STEP (JPMJTM20SL),
- JST J-RAPID (JPMJJR2007),
- JST SICORP (e-ASIA) (JPMJSC20U1),
- JST SICORP (JPMJSC21U5),
- JST CREST (JPMJCR20H6; JPMJCR20H4),
- JSPS KAKENHI Grant-in-Aid for Scientific Research (B) (18H02662; 21H02737),
- JSPS KAKENHI Grant-in-Aid for Scientific Research on Innovative Areas (16H06429; 16K21723; 17H05823; 17H05813; 19H04843; 19H04826),
- JSPS Fund for the Promotion of Joint International Research (Fostering Joint International Research) (18KK0447),
- JSPS Core-to-Core Program (JPJSCCB20190009),
- JSPS Core-to-Core Program (A. Advanced Research Networks) (JPJSCCA20190008),
- JSPS Research Fellow DC1 (19J20488),
- JSPS Leading Initiative for Excellent Young Researchers (LEADER),
- ONO Medical Research Foundation,
- Ichiro Kanehara Foundation,
- Lotte Foundation,
- Mochida Memorial Foundation for Medical and Pharmaceutical Research,
- Daiichi Sankyo Foundation of Life Science,
- Sumitomo Foundation,
- Uehara Foundation,
- Takeda Science Foundation,
- The Tokyo Biochemical Research Foundation,
- Mitsubishi Foundation,
- Shin-Nihon Foundation of Advanced Medical Research,
- Kumamoto University COVID-19 Research Projects (AMABIE),
- Kumamoto University International Collaborative Research Grants,
- Intercontinental Research and Educational Platform Aiming for Eradication of HIV/AIDS,
- 2020 Tokai University School of Medicine Research Aid,
- Joint Usage/Research Center Program of Institute for Frontier Life and Medical Sciences at Kyoto University